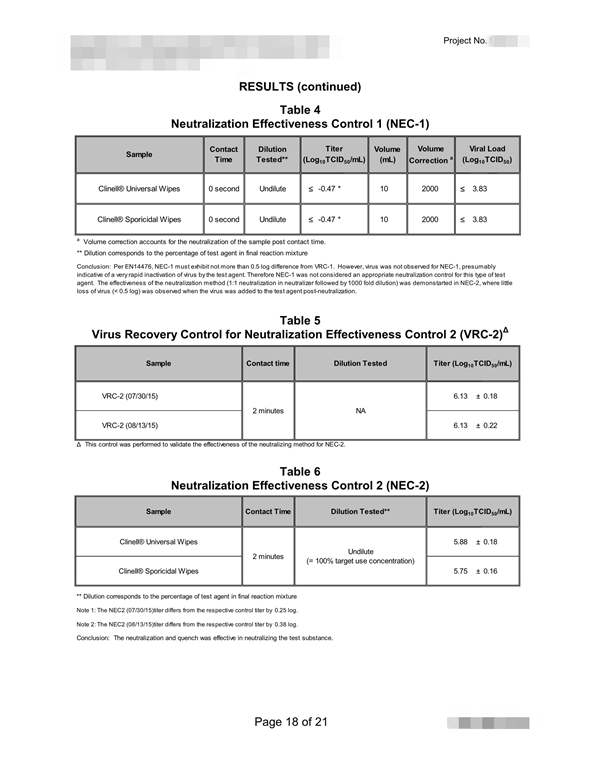
| EN14476, EN12791, 病毒杀灭测试 - 消毒剂, 防腐剂, 洗涤剂, 杀菌剂, 湿巾 | |||
| 脊髓灰质炎病毒杀灭试验, 150ml x 6pcs | |||
| 流感H1N1病毒杀灭试验, 150ml x 6pcs | |||
| 流感H3N2病毒杀灭试验, 150ml x 6pcs | |||
| 疱疹病毒杀灭试验, 150ml x 6pcs | |||
| 狂犬病毒杀灭试验, 150ml x 6pcs | |||
| 腺病毒灭活试验, 150ml x 6pcs | |||
| 手足口病毒杀灭试验, 150ml x 6pcs | |||
| 冠状病毒杀灭试验(229E型 , 150ml x 6pcs |
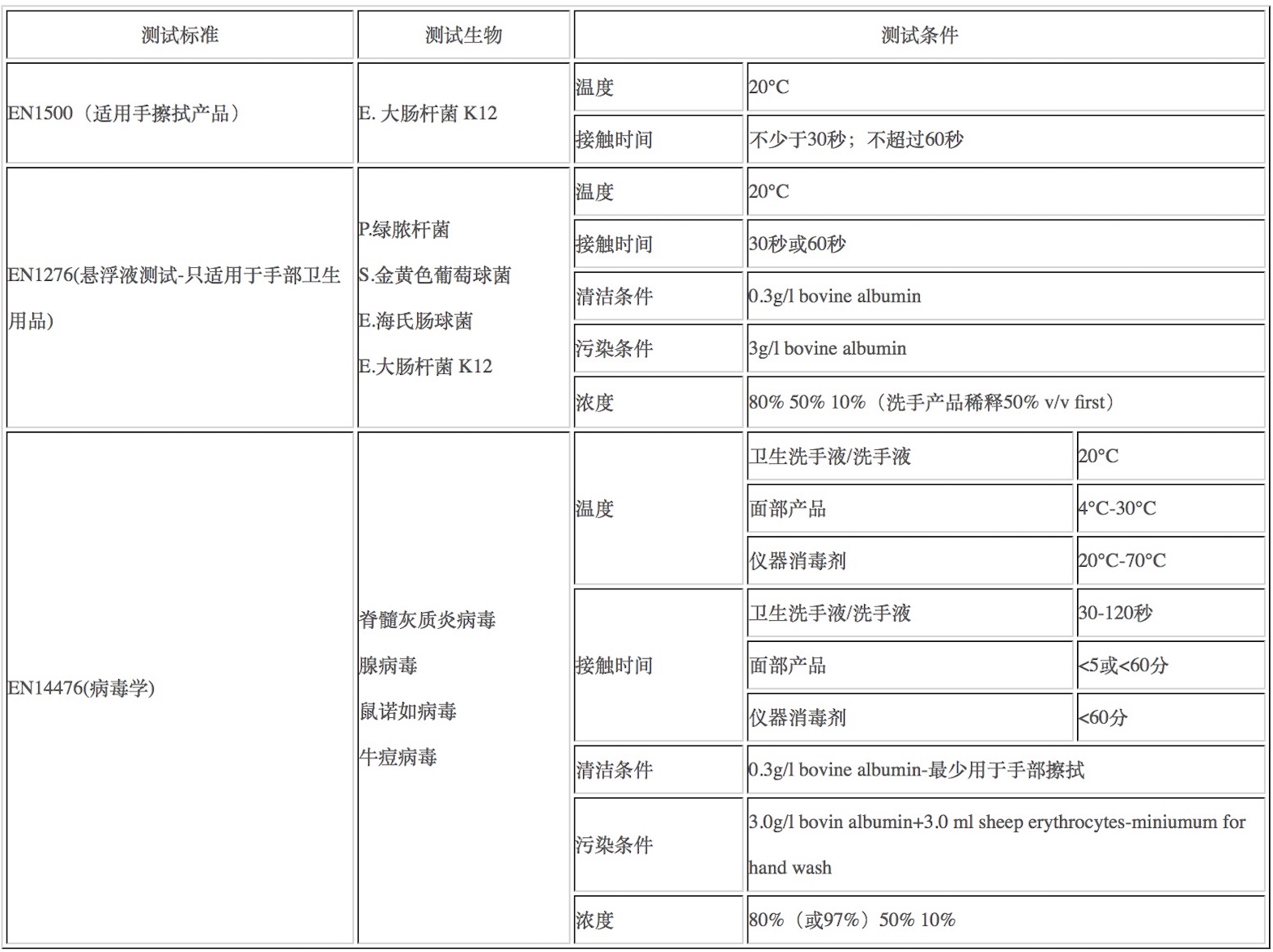
Nrom refer 涉及标准: EN 1276, EN 1040, EN 14476, ISO 21702, ISO 18184, ASTM E 2276, ASTM E 1054, ASTM E 1053, ASTM E 1052, ISO 21702, EN 1500, EN 1499, ASTM E 2784, ASTM E 1174, ASTM E 2755, ASTM E 1173, ASTM E 1115, ASTM E 2783, ASTM E 2752, ASTM E 2011, ASTM E 1838

英国实验室测试详解:
CORONAVIRUS 冠状病毒
Viruses 病毒: Influenza A virus H1N1 甲型H1N1流感病毒
COST/product (Low soil) 低浓度: RMBxxxx
COST/product (High soil) 高浓度: RMBxxxx
ENVELOPED VIRUSES 包膜病毒
Viruses 病毒: Vaccinia virus 痘苗病毒
COST/product (Low soil) 低浓度: RMBxxxx 此测试享有欧盟生物杀灭BPR法规官方授权
COST/product (High soil) 高浓度: RMBxxxx, 此测试享有欧盟生物杀灭BPR法规官方授权
CORONAVIRUS 冠状病毒
Viruses 病毒: Feline coronavirus OR Bovine coronavirus 猫冠状病毒或是牛冠状病毒
COST/product (Low soil) 低浓度: RMBxxxx
COST/product (High soil) 高浓度: RMBxxxx
以上报价不含样品快递至英国的费用,客户需要额外承担
Test conclusion 测试结论: fail; intermediate pass/fail; pass concentrations
Contact time 接触时间:
<2 minutes, hand sanitiser <2分钟, 洗手液
<5 minutes, Surface disinfectant, <5分钟, 表面消毒液
Contact temp 接触温度: 20 °C
Soil load 介质负荷:
Low, 0.3 g/l bovine albumin (Hand rub) 低,0.3 g/l牛白蛋白(手搓)OR
High, 3.0 g/l bovine albumin + 3.0 ml/L sheep erythrocytes (Hand wash) 高,3.0 g/l牛白蛋白+3.0 ml/l羊红细胞(洗手)
Pass criteria 通过标准: 4 log10 reduction with control validation. 4 log10 衰减控制验证。
10 weeks turn-around time 当前预估周期10周,最终周期以实验室收到样品后当天通知周期为准
Enveloped viruses 可选的包膜病毒
Poxviridae 痘病毒科
Herpesviridae 疱疹病毒科
Filoviridae (e.g. Ebola, Marburg) Flavivirus 丝状病毒科(如埃博拉、马尔堡)黄病毒
Hepatitis C Virus (HCV) 丙型肝炎病毒
Hepatitis Delta Virus (HDV) 三角型肝炎病毒
Influenza Virus 流感病毒
Paramyxoviridae 副粘病毒科
Rubella Virus 风疹病毒
Measles Virus 麻疹病毒
Rabies Virus 狂犬病病毒
Coronavirus (e.g. SARS, MERS) Human Immunodeficiency Virus (HIV) Human T Cell Leukemia Virus (HTLV) Hepatitis B virus (HBV) 冠状病毒(如SARS、MERS)、人类免疫缺陷病毒(HIV)、人类T细胞白血病病毒(HTLV)和乙型肝炎病毒(HBV)
All test samples must be accompanied by an MSDS. 所有客户提供的样品必须随附MSDS
Please provide a VAT number (for clients outside the UK) 英国境外客户必须提供税号
西班牙实验室测试详解:
GLP实验室资质
更多未注明病毒种类测试资料,需要联系实验室确认
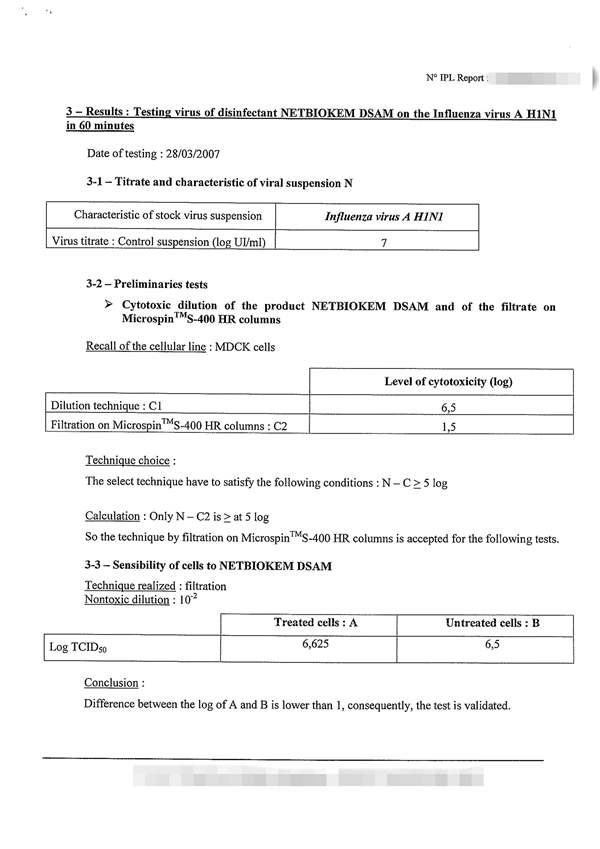
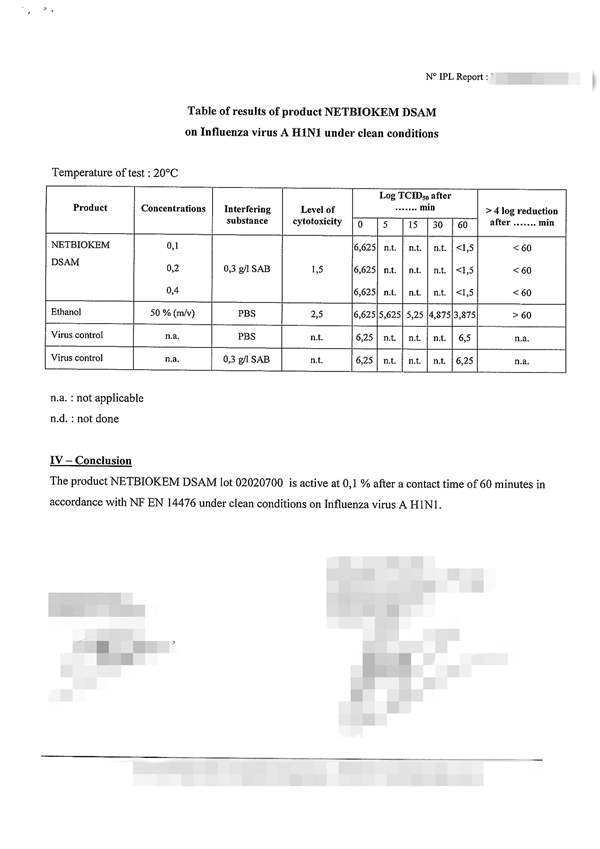
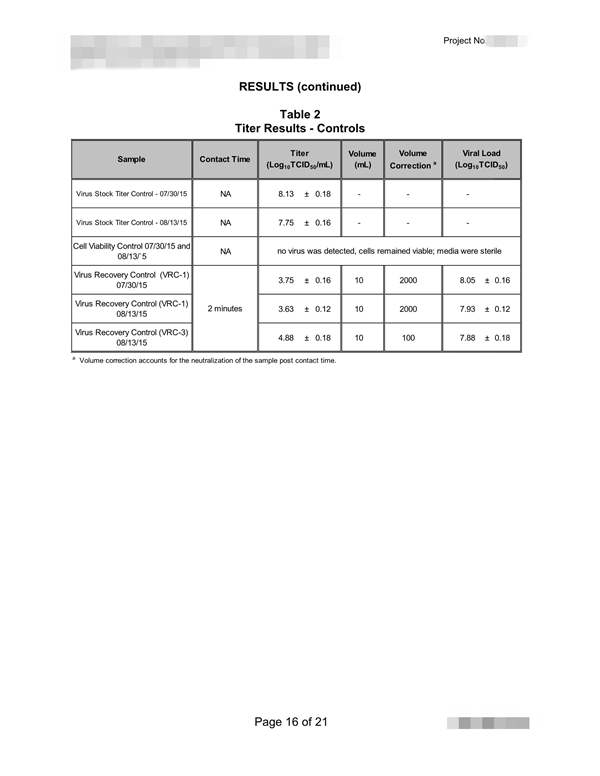
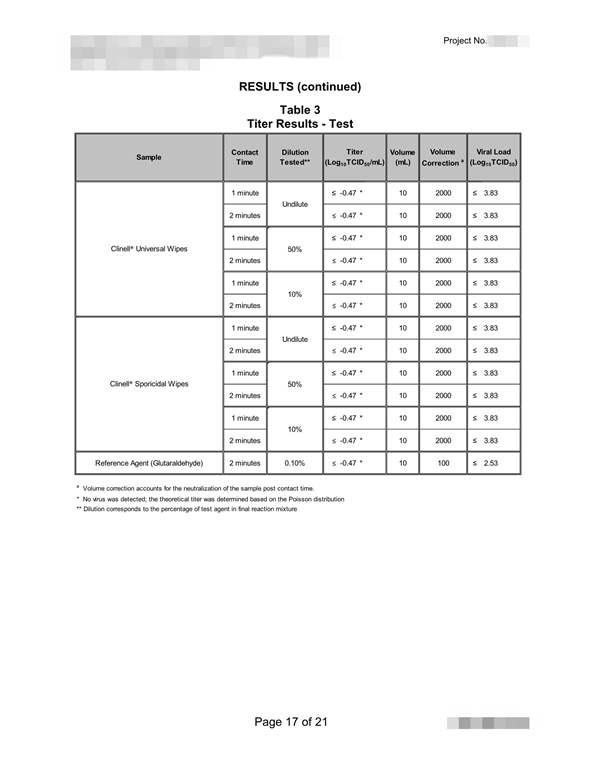
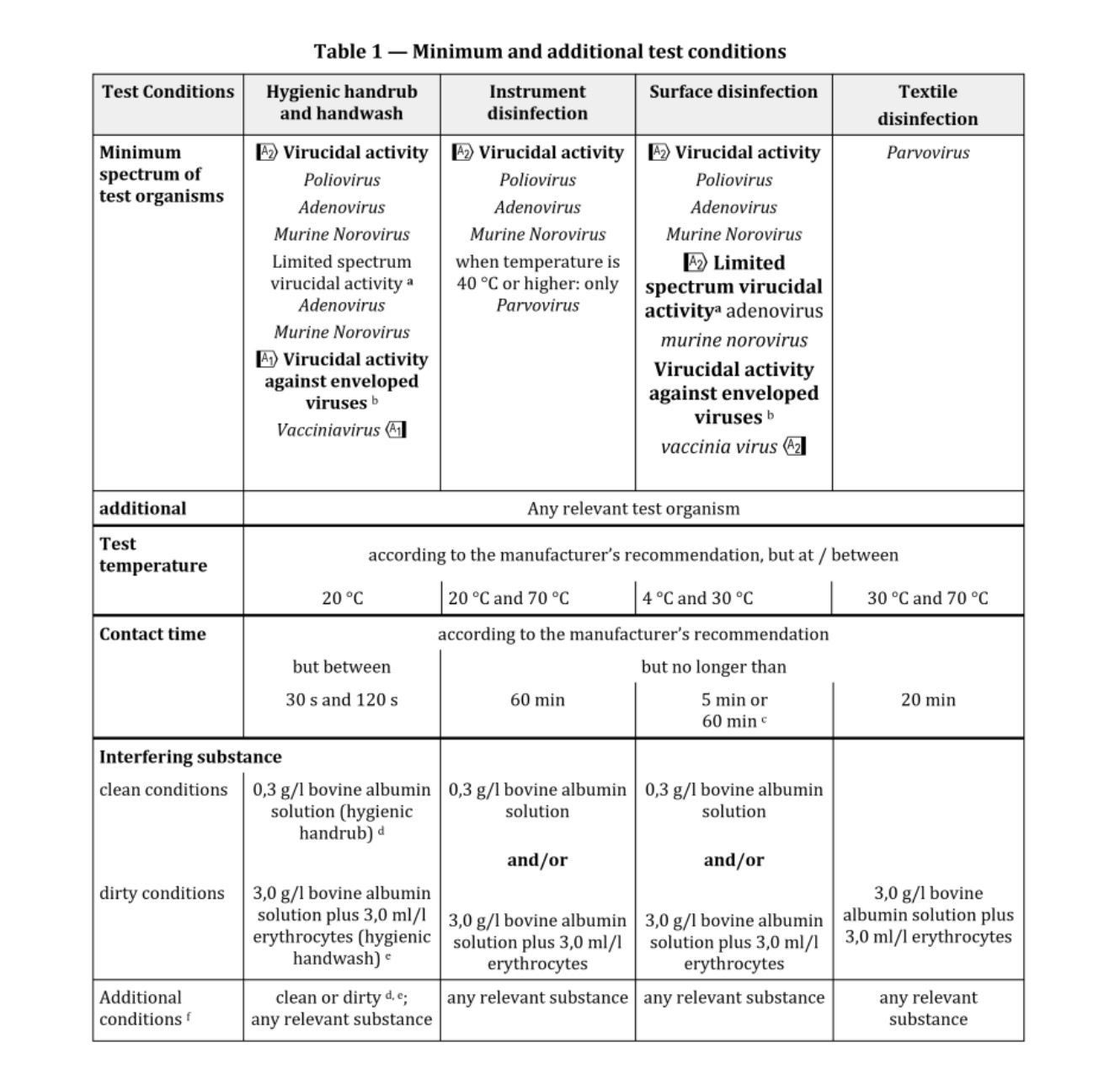
The NF EN 14476: 2013 + A2: 2019 standard includes the possibility of using the following viruses depending on the purpose of the test: 根据试验目的,NF EN 14476:2013+A2:2019标准包括使用以下病毒的可能性:
Poliovirus, Adenovirus, Murine Norovirus, Vaccinia virus and Parvovirus. The mandatory viruses required according to the intended application of the product and the mandatory conditions of the test are the following: 脊髓灰质炎病毒、腺病毒、小鼠诺如病毒、痘苗病毒和细小病毒。根据产品的预期用途和试验的强制性条件,所需的强制性病毒如下:
1) Hygienic handwashing or hygienic handrub: Poliovirus, Adenovirus and Murine Norovirus must be used for the general virucidal activity; only Adenovirus and Murine Norovirus when a limited spectrum against enveloped viruses and against certain viruses specified by the guideline: Norovirus, Adenovirus and Rotavirus; and only with Vaccinia virus when activity against enveloped viruses is desired*; assay temperature of 20oC; time of contact between 30 and 120 seconds*; and at least under clean conditions for hygienic handrub, or dirty conditions for hygienic handwashing*; 1)卫生洗手或卫生洗手:脊髓灰质炎病毒、腺病毒和小鼠诺如病毒必须用于一般病毒的杀灭活动;只有腺病毒和鼠诺如病毒在针对包膜病毒和指南规定的某些病毒的有限范围内才能使用:诺如病毒、腺病毒和轮状病毒;并且只有当需要对抗包膜病毒的活性时才使用疫苗病毒*;分析温度为20℃;接触时间在30到120秒之间*;并且至少在卫生洗手的清洁条件下,或在卫生洗手的肮脏条件下*;
2) Instruments disinfection: Poliovirus, Adenovirus and Murine Norovirus must be used, or only Parvovirus when temperature for disinfection is 40oC or higher*; assay temperature between 20 and 70oC*; time of contact not higher than 60 min; and under clean or dirty conditions*; 2) 仪器消毒:必须使用脊髓灰质炎病毒、腺病毒和小鼠诺如病毒,或仅在消毒温度为40℃或更高的情况下使用细小病毒*;分析温度在20℃到70℃之间*;接触时间不超过60分钟;在干净或肮脏的条件下*;
3) Surface disinfection: Poliovirus, Adenovirus and Murine Norovirus must be used for the general virucidal activity; only Adenovirus and Murine Norovirus when a limited spectrum against enveloped viruses and against certain viruses specified by the guideline: Norovirus, Adenovirus and Rotavirus; and only with Vaccinia virus when activity against enveloped viruses is desired*; assay temperature between 4 and 30oC*; time of contact of 5 min maximum, or 60 min for surfaces next to patients*; and under clean or dirty conditions*; 3) 表面消毒:脊髓灰质炎病毒、腺病毒和小鼠诺如病毒必须用于一般病毒的杀灭;只有腺病毒和小鼠诺如病毒,当对包膜病毒和指南中规定的某些病毒有一定的抑制作用时:诺如病毒、腺病毒和轮状病毒;只有痘苗病毒在对抗病毒时才使用需要包膜病毒*;分析温度在4到30℃之间*;接触时间最多5分钟,或者病人旁边的表面接触时间为60分钟*;在干净或肮脏的条件下*;
4) Tissue or clothes disinfection: Parvovirus; assay temperature between 30 and 70oC*; time of contact not higher than 20 min*, and under obligate dirty conditions. The product must be assayed at least at three concentrations, including one in the non-active concentration range and another in the active concentration range (these two last concentrations are selected by the testing laboratory). To consider the product as virucidal a 4 logarithmic reduction in the infectivity of viruses must be obtained. The cost with the basic conditions chosen by the manufacturer/customer, includes a test temperature; a contact time; a test concentration; and a test condition - clean or dirty-. If the customer wishes to evaluate the product in other conditions, in other contact times or in more concentrations chosen by him. (*: the customer must choose among the alternatives conditions and indicate the selected conditions in the test submission form).4) 纸巾或衣物消毒:细小病毒;检测温度在30到70摄氏度之间*;接触时间不超过20分钟*,在特定的肮脏条件下。产品必须至少进行三种浓度的化验,包括一种在非活性浓度范围内,另一种在活性浓度范围内(最后两种浓度由检测实验室选择)。病毒感染性的对数乘积必须考虑为4。制造商/客户选择的基本条件下的成本包括测试温度、接触时间、测试浓度和测试条件(干净或肮脏)。如果客户希望在其他条件下,在其他接触时间或他选择的更多浓度下评估产品。(*:客户必须在备选条件中进行选择,并在测试提交表格中注明所选条件)。
Important notes 重要提醒:
Concentration of the test product:
The products can be tested at a concentration equal to or less than 80%. Ready-to-use products can be tested at 97% if they do not pass the procedure at 80% in a test with three concentrations. In the 97% product test, the test conditions change, so it is done independently. For handwashing products, the maximum concentration in the assay is 50%.
试验品浓度:
产品可在等于或小于80%的浓度下进行试验。在三种浓度的试验中,如果现成产品在80%的条件下未通过程序,则可在97%的条件下进行试验。在97%的产品测试中,测试条件会发生变化,因此是独立进行的。对于洗手产品,分析中的最大浓度为50%。
Status of the test product:
Avoid sending the product in a gel state. Whenever possible, the product should be sent without the gelling agent. Otherwise, we cannot assure you that the test can be performed. This is because, in order to eliminate the cytotoxicity of the products, they are subjected to ultrafiltration in columns, a process that cannot be carried out in products of thick consistency such as gels.
试验产品状态:避免以凝胶状态发送产品。如有可能,产品应不含胶凝剂。否则,我们不能保证可以进行测试。这是因为,为了消除产品的细胞毒性,它们需要在柱中进行超滤,而这一过程不能在凝胶等稠度产品中进行。
Cytotoxicity of the test product:
For very cytotoxic products that require the use of 4 or more ultrafiltration columns to eliminate cytotoxicity, a supplement will be charged.
试验产品的细胞毒性:
对于需要使用4个或更多超滤柱来消除细胞毒性的非常具有细胞毒性的产品,将收取附加费
Cost of test with guideline mandatory viruses:
使用指导性强制病毒的测试:
Basic price with the three mandatory viruses (Poliovirus type 1, Adenovirus type 5 and Murine Norovirus)
三种强制性病毒(脊髓灰质炎病毒1型、腺病毒5型和小鼠诺如病毒)
Basic price with the three mandatory viruses according to the standard guideline for general virucidal activity (Poliovirus type 1, Adenovirus type 5 and Murine Norovirus) only with the 97% concentration, because no activity was obtained at 80% concentration previously tested
三种强制性病毒(脊髓灰质炎病毒1型、腺病毒5型和小鼠诺如病毒)97%浓度的基本价格,因为在之前测试的80%浓度下没有活性
Basic price with one of the mandatory viruses according to the standard guideline (Poliovirus typo 1, Adenovirus typo 5 or Murine Norovirus), only with the 97% concentration, because no activity was obtained at 80% concentration previously tested
根据标准指南,其中一种强制性病毒(脊髓灰质炎病毒1型、腺病毒5型或小鼠诺如病毒)97%的浓度的基本价格,因为在之前测试的80%浓度下没有活性
Basic price for testing with four concentrations (three guideline mandatory concentrations plus the 97%), with the three viruses indicated by the guideline (Poliovirus type 1, Adenovirus type 5 and Murine Norovirus), because it is necessary to follow an independent and different procedure for the 97% concentration
使用四种浓度(三种指导性强制性浓度加上97%)进行试验的基本价格,以及指南中指出的三种病毒(脊髓灰质炎病毒1型、腺病毒5型和小鼠诺如病毒),因为有必要对97%的浓度遵循一种独立和不同的程序
Basic price with only two viruses (any of the three mandatory viruses: Poliovirus type 1, Adenovirus type 5 or Murine Norovirus)
基本价格仅含两种病毒(三种强制病毒中的任意一种:脊髓灰质炎病毒1型、腺病毒5型或小鼠诺如病毒)
Basic price with only two viruses (Adenovirus type 5 or Murine Norovirus) for “limited antiviral activity” (Activity against enveloped viruses, + Adenovirus + Norovirus + Rotavirus)
“有限抗病毒活性”(针对包膜病毒,+腺病毒+诺如病毒+轮状病毒)的基本价格仅含两种病毒(腺病毒5型或小鼠诺如病毒
Basic price with only one virus (any of the three mandatory viruses: Poliovirus type 1, Adenovirus type 5 or Murine Norovirus)
只有一种病毒的基本价格(三种强制病毒中的任何一种:脊髓灰质炎病毒1型、腺病毒5型或小鼠诺如病毒)
Basic price with only Murine Parvovirus (textile disinfection)
基本价格仅含鼠细小病毒(纺织品消毒)
Basic price with Vaccinia virus for virucidal activity against enveloped viruses
牛痘病毒对包膜病毒杀灭活性的基本价格
Basic price for a screening test with the three mandatory viruses according to the standard
guideline for general virucidal activity (Poliovirus type 1, Adenovirus type 5 and Murine Norovirus) only with one concentration, without positive and negative test controls (the report will be issued without the ENAC accreditation logo)
三种强制性病毒按标准进行筛选试验的基本价格
一般病毒活性指南(脊髓灰质炎病毒1型,腺病毒5型和小鼠诺如病毒),只有一种浓度,没有阳性和阴性试验对照(报告将在没有ENAC认证标志的情况下发布)
Supplement when 4 or more ultrafiltration columns to eliminate cytotoxicity for cell culture must be used
当必须使用4个或更多超滤柱以消除细胞毒性时,必须使用细胞培养
- Note**: We can use any virus requested by the customers if the virus strain is available from Culture Collections (ATCC, NCPV or others). We try to obtain accreditation for those requested viruses not included in the guideline in the annual accreditation audit. In the meanwhile, for those optional viruses not mandatory by the standard guideline, to be under accreditation, the National Accreditation Entity (ENAC) requires performing the test at the same time with one the mandatory virus.
Note***: When test is performed with surrogate viruses is due to the one of the following situations: highly contagious viruses not recommended to handle in the laboratory for due to risk for the lab personnel, or problems for replication in cell culture as required by this type of tests.
Note****: Calculations of costs for more than one concentration, more than one time, more than one temperature and/or more than one conditions, chosen by the customer: multiply the basic cost by the number of variables and by 0.9.
Note *****: We perform efficacy test with Coronavirus, specifically with human Coronavirus 229E. However, we do not carry out the virucidal activity tests with SARS-CoV-2 (COVID-19 virus; 2019nCoV; Wuhan Coronavirus), the cause of the recent health alert, and we do not intend to do so due to the connotations that the mentioned virus has to produce human infections and because we consider it unnecessary to demonstrate the antiviral activity of a disinfectant product against this strain for the following reasons.
Coronaviruses (any of them), are enveloped viruses, and for this reason they are very sensitive to many disinfectants. In addition, all Coronaviruses have the same viral structure, and therefore, they have the same sensitivity to disinfectants. If some of them produce more human pathology than others, it is because they interact with host cell receptors differently. The new Coronaviruses that have been described are a consequence of the adaptation of animal Coronaviruses (in these cases viruses of bats, which have adapted to humans, in general with an intermediate stage by other mammals -civet and others-, hence they have been infected in the animal markets of Asia -SARSCoV-, and of bats through camelids the MERSCoV).
注**:如果从培养物收集(ATCC、NCPV或其他)获得病毒株,我们可以使用客户要求的任何病毒。我们尝试对那些未列入年度认证审核指南的病毒进行认证。同时,对于那些标准指南中没有强制要求的可选病毒,国家认证机构(ENAC)要求与一个强制性病毒同时进行测试。 - 注***:当使用替代病毒进行试验时,是由于以下情况之一:由于实验室人员的风险,不建议在实验室处理高传染性病毒,或此类试验要求的细胞培养复制问题。
- 注****:客户选择一种以上浓度、一次以上、一种以上温度和/或多种条件的成本计算:将基本成本乘以变量数,再乘以0.9(。
- 注*****:我们对冠状病毒进行疗效测试,特别是对人类冠状病毒229E进行疗效测试。但是,我们不使用引起最近健康警报的SARS-CoV-2(COVID-19病毒;2019nCoV;武汉冠状病毒)进行病毒活性测试,由于上述病毒必须产生的含义,我们不打算这样做因为我们认为没有必要证明消毒剂对这种病毒株的抗病毒活性,原因如下。
- 冠状病毒(任何一种)都是包被病毒,因此它们对许多消毒剂非常敏感。此外,所有冠状病毒都有相同的病毒结构,因此,它们对消毒剂的敏感性相同。如果它们中的一些比其他的产生更多的人类病理学,那是因为它们与宿主细胞受体的相互作用不同。所描述的新冠状病毒是动物冠状病毒适应的结果(在这种情况下,蝙蝠的病毒已经适应人类,一般来说是其他哺乳动物——灵猫和其他哺乳动物——的中间阶段——因此,它们在亚洲的动物市场——萨尔斯科夫——和蝙蝠通过骆驼类感染梅尔斯科夫)



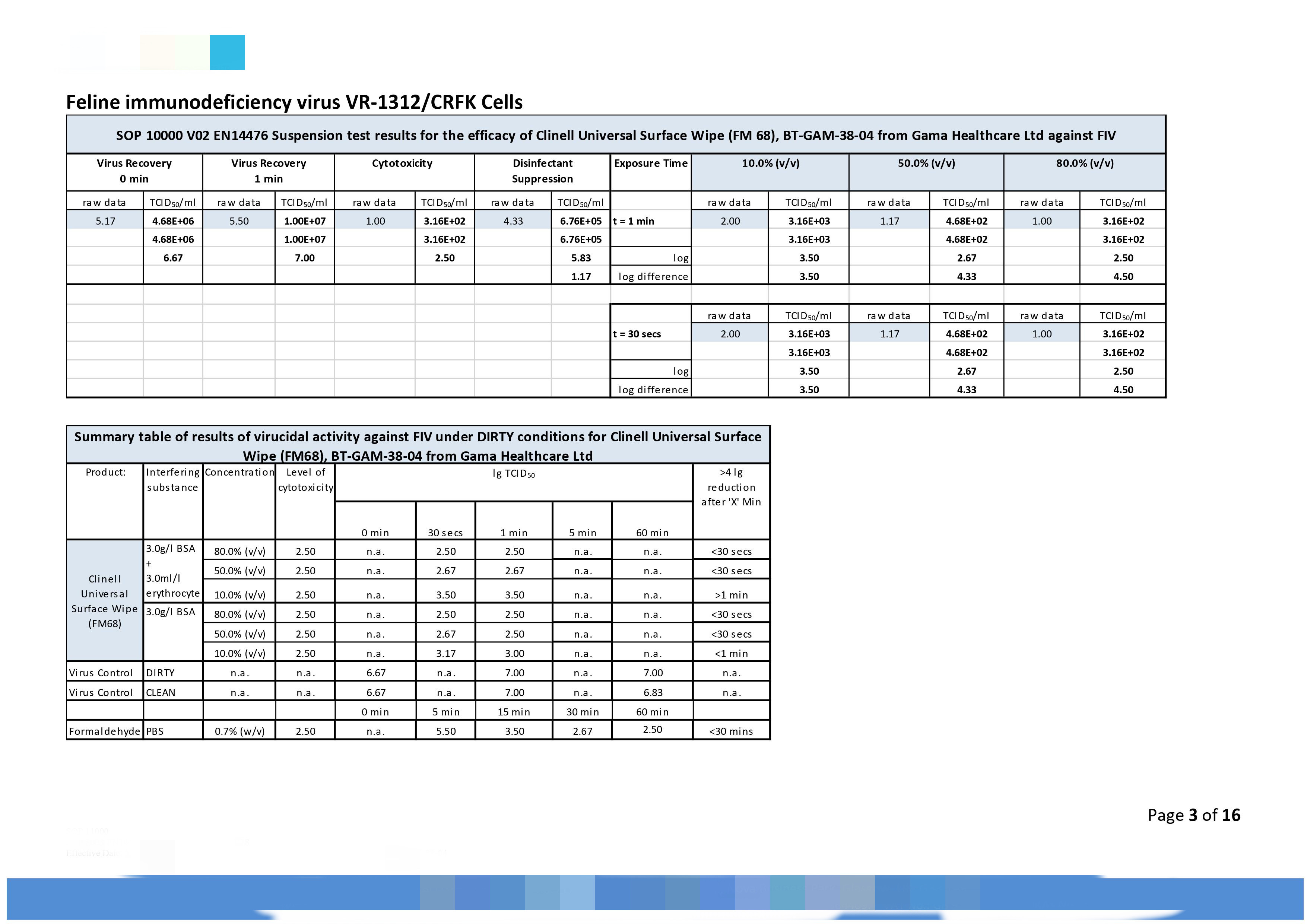
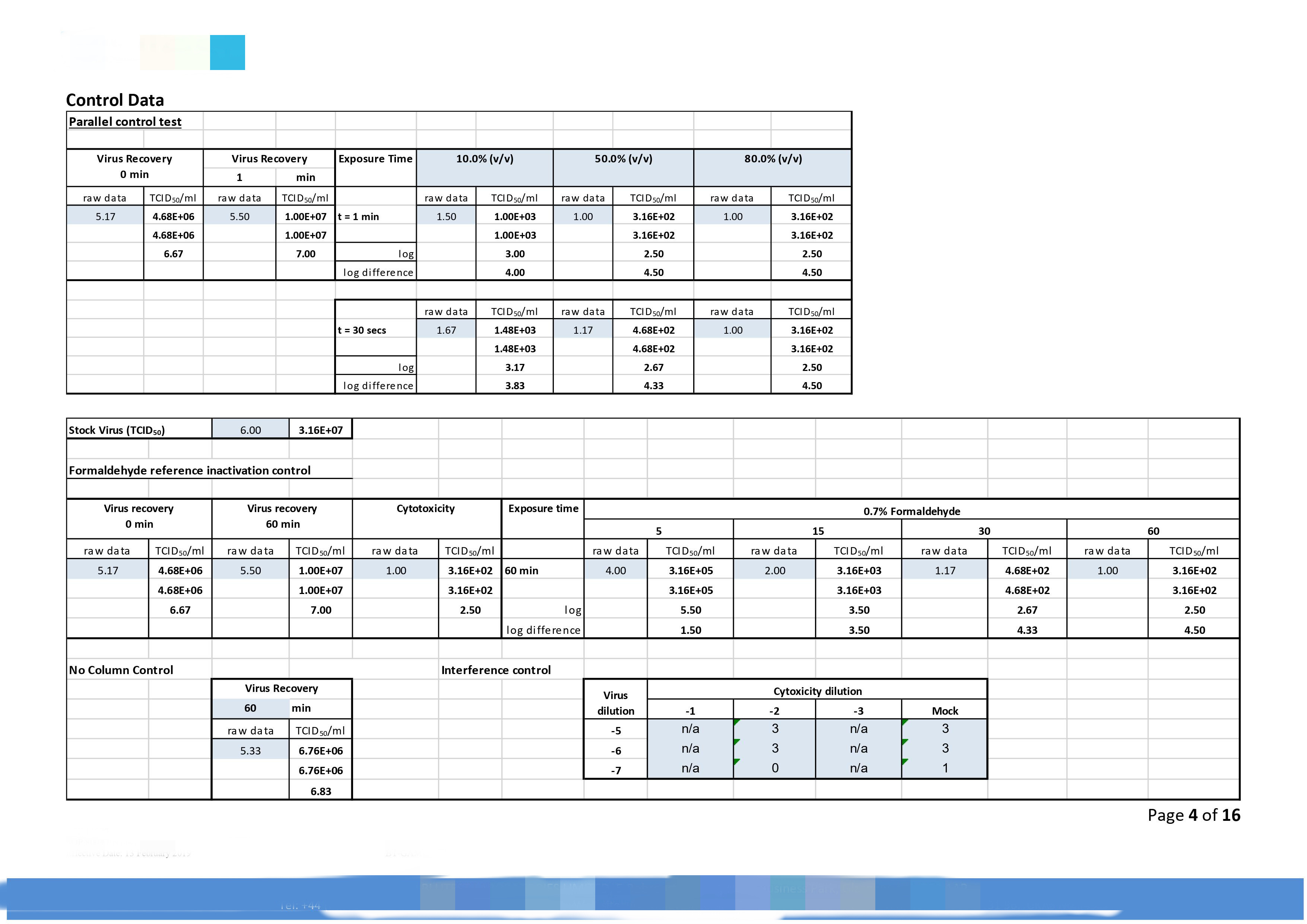
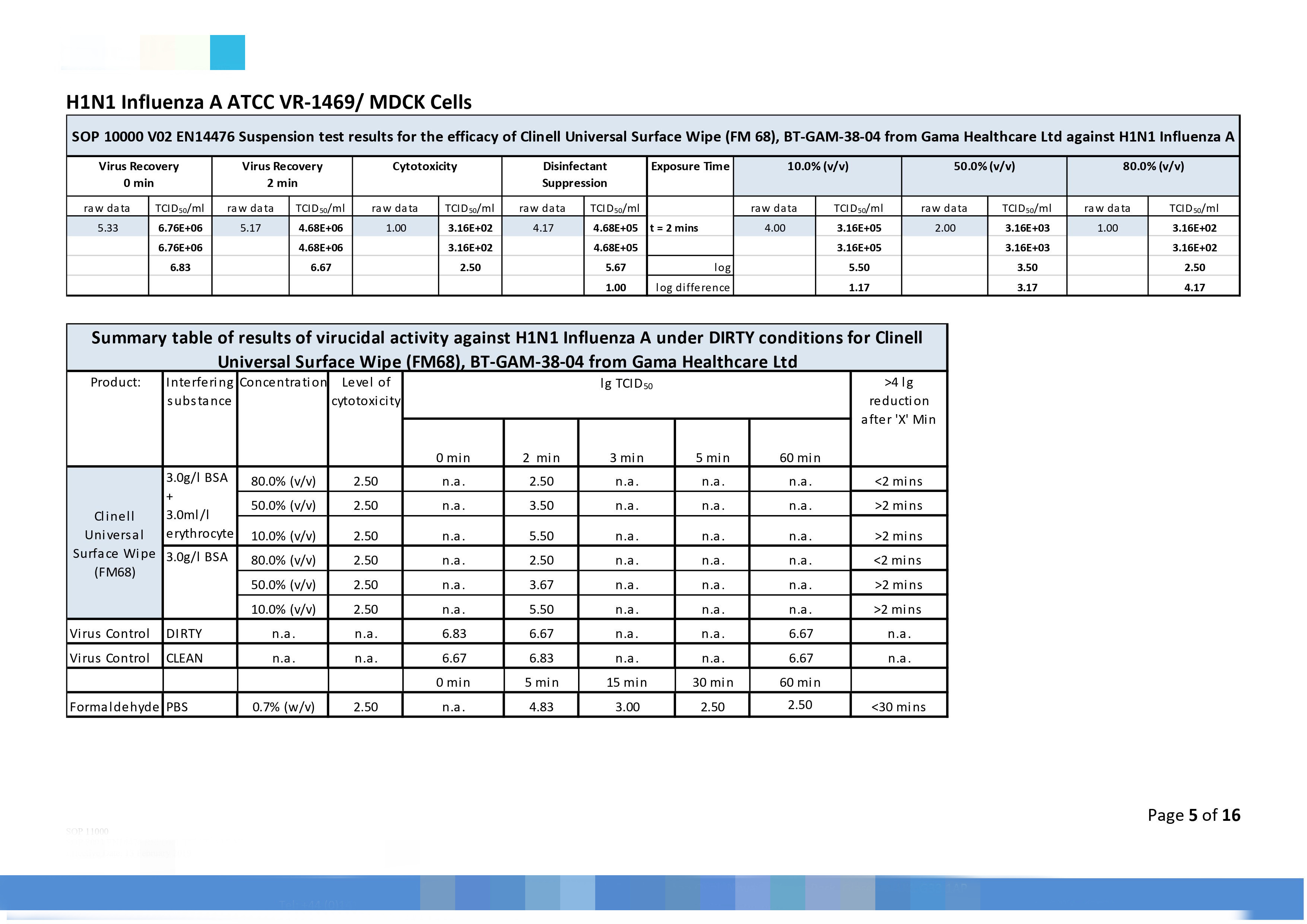
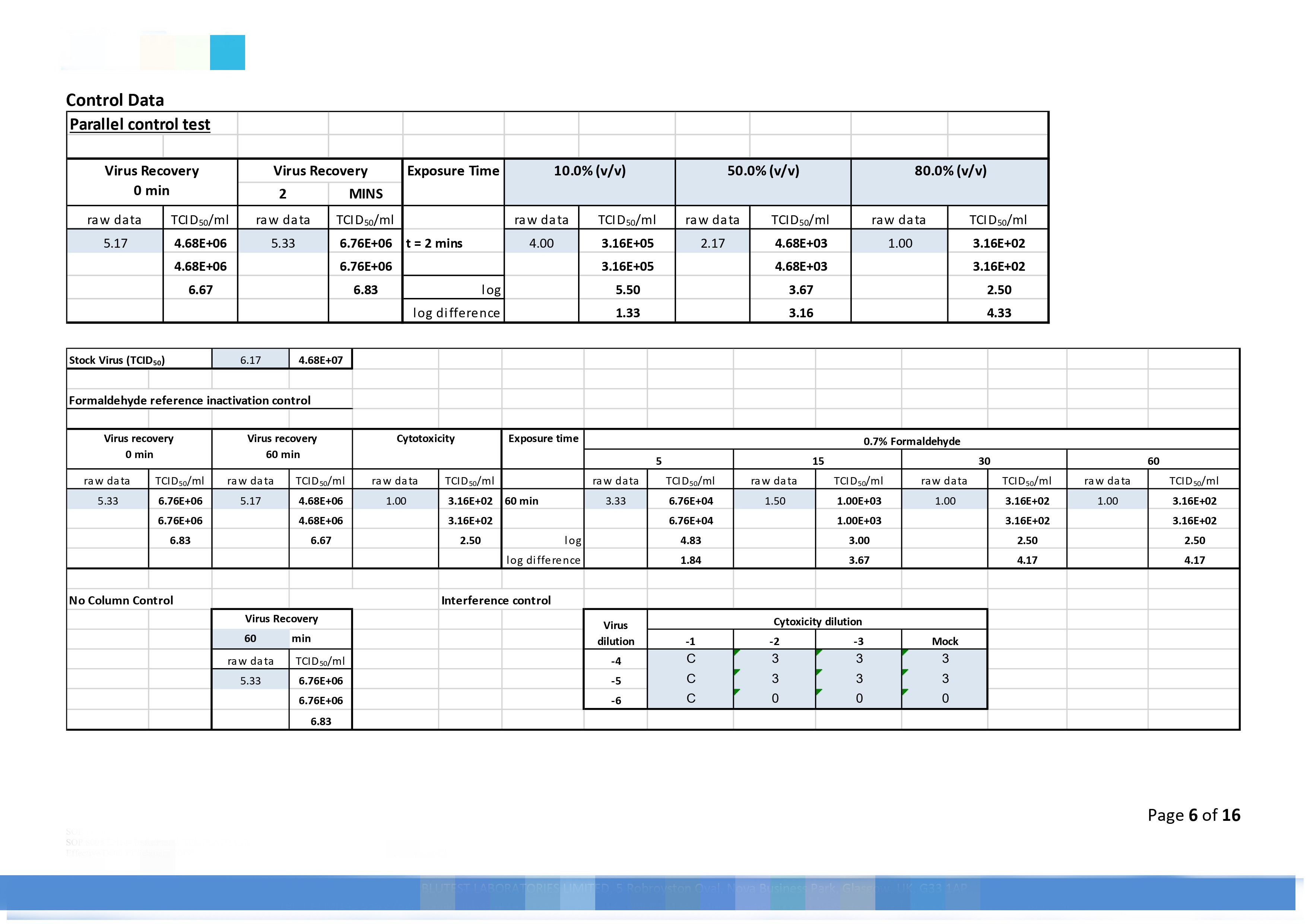
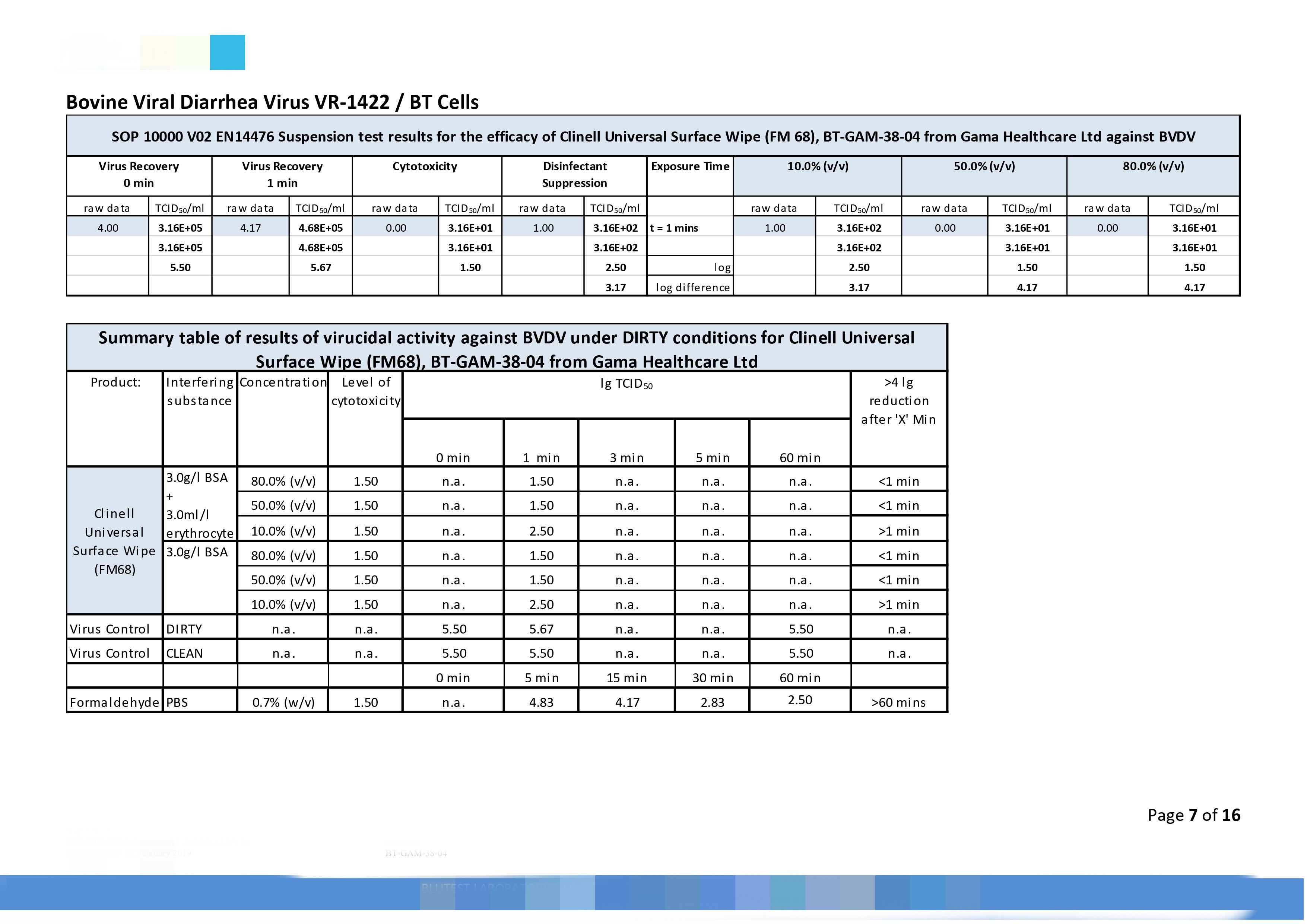
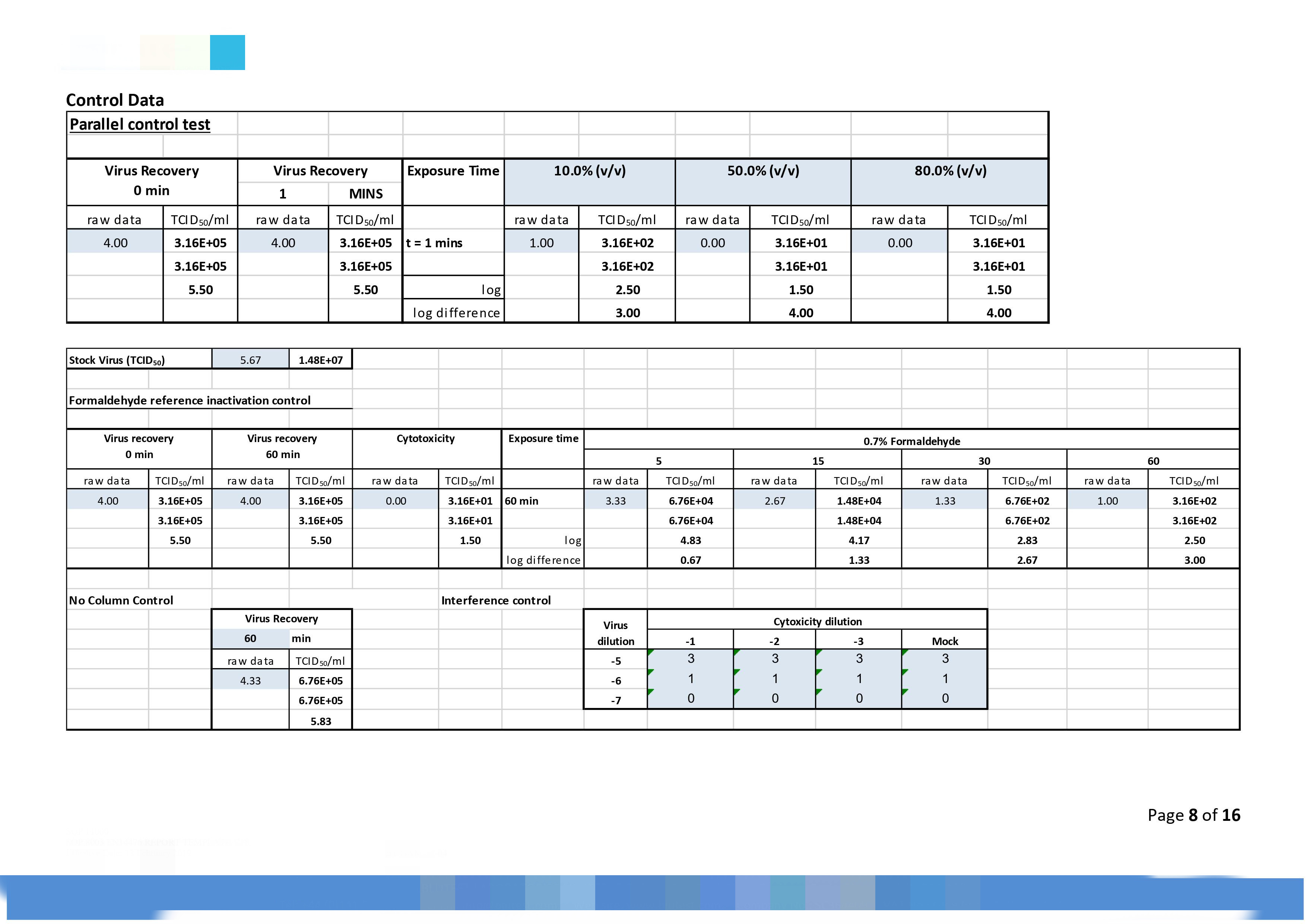
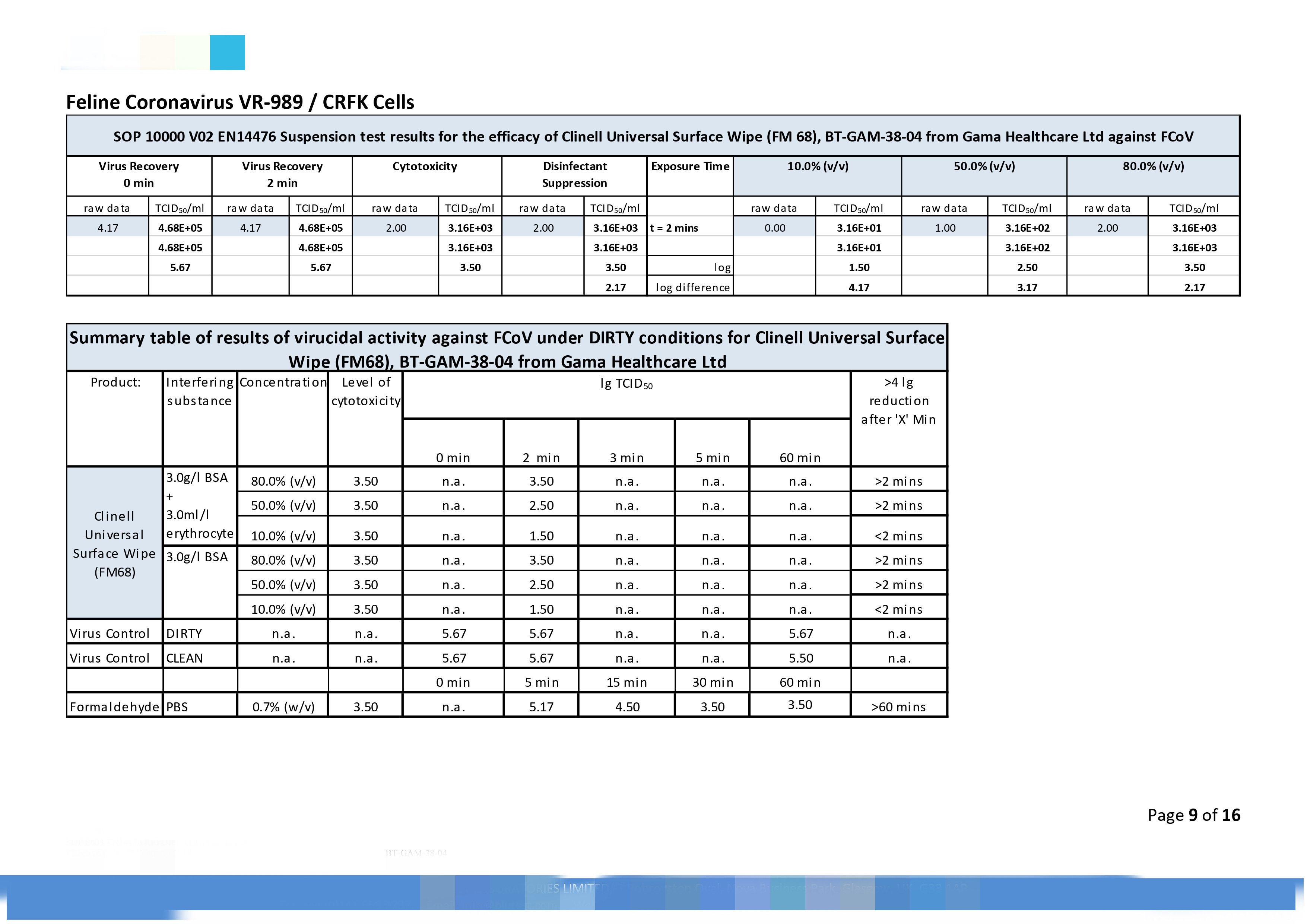
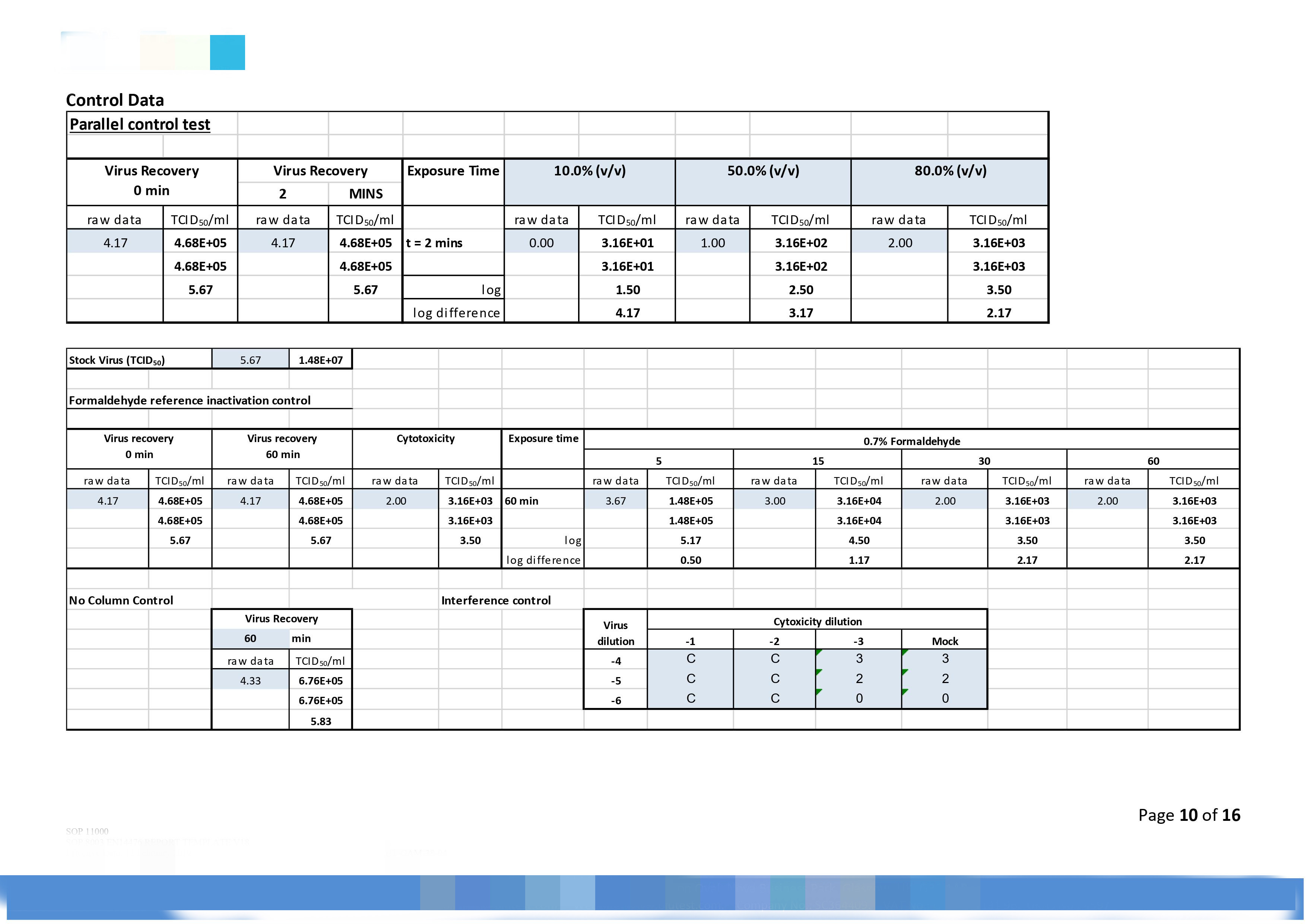
|
The information shown in this guideline document is for information only and provides the standard test conditions to assist you in completing the sample submission form. |
||
|
MEDICAL AREA: Bacteria, Fungi, Mycobacteria, Virus.
NOTE: Hand wash products are diluted to 50.0% as standard |
||
|
STANDARD |
TEST ORGANISMS |
STANDARD CONDITIONS |
|
EN 13624:
|
Suspension test |
C. albicans
A. brasiliensis |
Temperature |
Hygienic Handrub |
20OC |
|
Surgical Handrub |
20OC |
||||
|
Surface product |
4OC- 30OC |
||||
|
Instrument disinfectant |
20OC- 70OC |
||||
|
Contact time |
Hygienic Handrub |
30 secs- 60 secs |
|||
|
Surgical Handrub |
1 min- 5 mins |
||||
|
Surface product |
≤ 5 minutes |
||||
|
Instrument disinfectant |
≤ 60 minutes |
||||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
80% 50% 10% |
||||
|
EN 13727:
|
Suspension test |
P. aeruginosa
S. aureus
E. hirae
E. coli K12 (Hand products only) |
Temperature |
Hygienic Handrub |
20OC |
|
Surgical Handrub |
20OC |
||||
|
Surface product |
4OC- 30OC |
||||
|
Instrument disinfectant |
20OC- 70OC |
||||
|
Contact time |
Hygienic Handrub |
30 secs- 60 secs |
|||
|
Surgical Handrub |
1 min- 5 mins |
||||
|
Surface product |
≤5 minutes |
||||
|
Instrument disinfectant |
≤60 minutes |
||||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
80% (or 97%) 50% 10% |
||||
|
*10% cost increase on tests performed at 97% |
|||||
|
EN 14348:
|
Suspension test |
M. avium
M. terrae |
Temperature |
20OC |
|
|
Contact time |
Instrument disinfectant |
<60 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
80% 50% 10% |
||||
|
EN 14476:
VIROLOGY
|
Suspension test |
Poliovirus-1
Adenovirus-5
Murine norovirus Vaccinia Virus |
Temperature |
Hygienic Handrub/hand wash |
20OC |
|
Surface product |
4OC- 30OC |
||||
|
Instrument disinfectant |
20OC- 70OC |
||||
|
Contact time |
Hygienic Handrub/hand wash |
30 – 120 seconds |
|||
|
Surface product |
<5 or <60 minutes |
||||
|
Instrument disinfectant |
<60 minutes |
||||
|
Clean conditions |
0.3 g/l bovine albumin- minimum for hand rub |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes- minimum for hand wash |
||||
|
Concentrations |
80% (or 97%*) 50% 10% |
||||
|
*20% cost increase on tests performed at 97%. Does not include Murine norovirus and Vaccinia virus |
|||||
|
EN 14561:
|
Glass surface test |
P. aeruginosa
S. aureus
E. hirae |
Temperature |
20OC |
|
|
Contact Time |
Instrument disinfectant |
<60 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
100% 50% 10% |
||||
|
|
The information shown in this guideline document is for information only and provides the standard test conditions to assist you in completing the sample submission form. |
|||
|
|
MEDICAL AREA: Bacteria, Fungi, Mycobacteria, Virus.
NOTE: Hand wash products are diluted to 50.0% as standard |
|||
|
STANDARD |
TEST ORGANISMS |
STANDARD CONDITIONS |
|
|
|
EN 14562:
|
Glass surface test |
C. albicans
A. brasiliensis |
Temperature |
20OC |
|
|
Contact Time |
Instrument disinfectant |
<60 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
100% 50% 10% |
||||
|
EN 14563:
|
Glass surface test |
M. avium
M. terrae |
Temperature |
20OC |
|
|
Contact Time |
Instrument disinfectant |
<60 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
Concentrations |
100% 50% 10% |
||||
|
EN 16615:
|
Surface test |
P. aeruginosa
S. aureus
E. hirae
C. albicans
|
Temperature |
4OC - 30OC |
|
|
Contact Time |
No less than 1 minute, no greater than 60 minutes |
||||
|
Clean conditions |
0.3 g/l bovine albumin |
||||
|
Dirty conditions |
3.0 g/l bovine albumin + 3.0 ml sheep erythrocytes |
||||
|
The information shown in this guideline document is for information only and provides the standard test conditions to assist you in completing the sample submission form. |
||||
|
GENERAL PURPOSE AREA: Bacteria, Fungi, bacterial endospores, Hand rubs / washes
NOTE: Hand wash products are diluted to 50.0% as standard |
||||
|
STANDARD |
TEST ORGANISMS |
STANDARD CONDITIONS |
||
|
EN 1040:
|
Suspension test |
P. aeruginosa
S. aureus |
Temperature |
20OC |
|
Contact time |
5 minutes |
|||
|
Clean conditions |
None |
|||
|
Dirty conditions |
None |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1275:
|
Suspension test |
C. albicans
A. brasiliensis |
Temperature |
20OC |
|
Contact time |
15 minutes |
|||
|
Clean conditions |
None |
|||
|
Dirty conditions |
None |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1276:
|
Suspension test – |
P. aeruginosa
S. aureus
E. hirae
E. coli
E. faecium (temp ≥40°C) |
Temperature |
4°C - 60°C (typically 20°C) |
|
Contact time |
1 min - 60 mins (typically 5 mins) |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1276:
|
Suspension test – hand hygiene products only |
P. aeruginosa
S. aureus
E. hirae
E. coli K12 |
Temperature |
20°C |
|
Contact time |
30 s or 60 s |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
80% 50% 10% (handwash products diluted 50% v/v first) |
|||
|
EN 1650:
|
Suspension test |
C. albicans
A. brasiliensis |
Temperature |
4°C - 40°C (typically 20°C) |
|
Contact time |
1 min - 60 mins (typically 15 mins) |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1650:
|
Suspension test – hand hygiene products only |
C. albicans
|
Temperature |
20°C |
|
Contact time |
30 s or 60 s |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
80% 50% 10% (handwash products diluted 50% v/v first) |
|||
|
EN 13704:
|
Suspension test |
B. subtilis |
Temperature |
20OC |
|
Contact time |
60 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
N/A |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 13697:
(Bacteria) |
Surface test |
P. aeruginosa
S. aureus
E. hirae
E. coli |
Temperature |
20OC |
|
Contact time |
5 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin/ 8.5g/lskimmed milk in distilled water (for P. aeruginosa only) |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
100% 50% 10% |
|||
|
EN 13697:
(Fungi) |
Surface test |
C. albicans
A. brasiliensis |
Temperature |
20OC |
|
Contact time |
15 minutes |
|||
|
Clean conditions |
0.3 g/l bovine albumin |
|||
|
Dirty conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
100% 50% 10% |
|||
|
The information shown in this guideline document is for information only and provides the standard test conditions to assist you in completing the sample submission form. |
|
|||||
|
GENERAL PURPOSE AREA (continued): Bacteria, Fungi, bacterial endospores, Hand rubs / washes
NOTE: Hand wash products are diluted to 50.0% as standard |
|
|||||
|
|
EN 1499:
|
Hand trial – Hand wash products |
E. coli K12 |
Temperature |
20OC |
|
|
|
Contact time |
No less than 30 seconds:
No more than 60 seconds |
||||
|
|
EN 1500:
|
Hand trial – Hand rub products |
E. coli K12 |
Temperature |
20OC |
|
|
|
Contact time |
No less than 30 seconds:
No more than 60 seconds |
||||
|
The information shown in this guideline document is for information only and provides the standardtest conditions to assist you in completing the sample submission form. |
||
|
VETERINARY AREA: Bacteria, Fungi, Mycobacteria, Virus |
||
|
STANDARD |
TEST ORGANISMS |
STANDARD CONDITIONS |
|
EN 1656:
|
Suspension test |
P. aeruginosa
S. aureus
E. hirae
P. vulgaris |
Temperature |
10OC |
|
Contact time |
30 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1656:
Teat Disinfectant Only |
Suspension test |
E. coli
S. aureus
S. uberis |
Temperature |
30OC |
|
Contact time |
5 minutes |
|||
|
Soiling conditions |
10.0 g/l skimmed milk |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1657:
|
Suspension test |
C. albicans
A. brasiliensis |
Temperature |
10OC |
|
Contact time |
30 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 1657:
Teat Disinfectant Only |
Suspension test |
C. albicans
|
Temperature |
30OC |
|
Contact time |
5 minutes for post milking
10 minutes for premilking |
|||
|
Soiling conditions |
10.0 g/l skimmed milk |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 14204:
|
Suspension test |
M. avium |
Temperature |
10OC |
|
Contact time |
60 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 14675:
VIROLOGY
|
Suspension test |
Bovine enterovirus-1
(ECBO) |
Temperature |
10OC |
|
Contact time |
30 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
80% 50% 10% |
|||
|
EN 14349:
|
Surface test |
P. aeruginosa
S. aureus
E. hirae
P. vulgaris |
Temperature |
10OC |
|
Contact time |
30 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
100% 50% 10% |
|||
|
EN 16437:
|
Surface test |
P. aeruginosa
S. aureus
E. hirae
P. vulgaris |
Temperature |
10OC |
|
Contact time |
60 minutes |
|||
|
Soiling Conditions |
3.0 g/l bovine albumin |
|||
|
Concentrations |
100% 50% 10% |
|||
|
EN 16438:
|
Surface test |
C. albicans
A. brasiliensis |
Temperature |
10OC |
|
Contact time |
60 minutes |
|||
|
Clean conditions |
3.0 g/l bovine albumin |
|||
|
Dirty conditions |
10.0 g/l bovine albumin + 10.0 g/l yeast extract |
|||
|
Concentrations |
100% 50% 10% |